Leadership Development
Build Critical Skills to Deploy Strategy in GMP Environments
We develop pharmaceutical manufacturing leaders—providing the necessary skills to deploy strategy in highly regulated operations. Our framework identifies critical skills and pinpoints what matters most at each leadership level.
The Leadership Development Gap
Pharmaceutical manufacturers promote technical experts into leadership roles, but rarely provide the targeted skill development needed to deploy strategy and drive operational excellence in GMP environments.
Leaders Lack Critical Skills
Front-line supervisors and managers are promoted for technical expertise, but lack the leadership skills needed to deploy strategy and drive operational excellence.
Generic Leadership Training
Most leadership development programs aren't designed for pharmaceutical manufacturing—they don't address GMP compliance, regulatory pressure, or batch manufacturing complexity.
No Clear Development Path
Organizations lack a structured framework for identifying which leadership skills matter most at each level—from front-line to functional to business unit leadership.
Leadership Development by Level
Our framework identifies critical skills for three leadership levels—ensuring development efforts focus on the capabilities that matter most for each role.
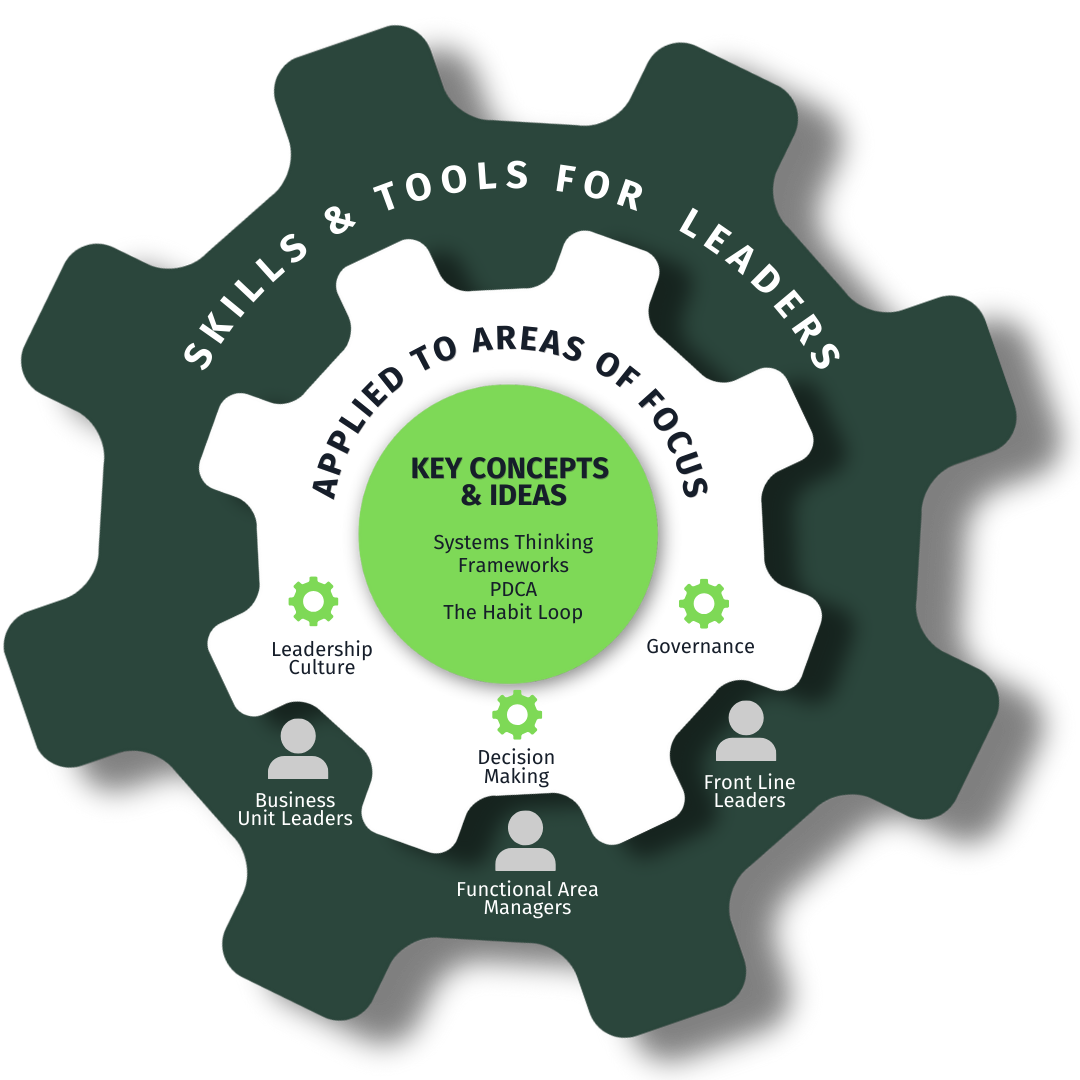
Front-Line Leaders
Supervisors and team leads who manage day-to-day batch manufacturing operations and quality oversight.
Critical Skills:
- Real-time problem solving and escalation
- Standard work adherence and coaching
- Daily performance management
- Cross-functional communication
- Safety and quality mindset
Functional Managers
Mid-level managers in charge of a function such as upstream manufacturing, downstream manufacturing, or support operations.
Critical Skills:
- Strategic thinking and priority setting
- Resource allocation and constraint management
- Performance system design
- Cross-functional collaboration
- Change management and improvement culture
Business Unit Leaders
Leaders who own multiple functions across the manufacturing organization.
Critical Skills:
- Strategy deployment and cascading goals
- Organizational design and capability building
- Stakeholder management and influence
- Risk assessment and decision-making
- Culture transformation and leadership development
Our Leadership Development Framework
GMPKit's leadership development toolset is specifically designed to build the skills needed to deploy strategy in highly regulated pharmaceutical operations.
Skill Identification
We identify the critical leadership skills needed to deploy strategy in highly regulated pharmaceutical operations—from front-line execution to business unit leadership.
Level-Specific Focus
We pinpoint which skills are most used at each leadership level—ensuring development efforts focus on the capabilities that matter most for each role.
Contextual Insights
Our materials provide insights into each skill: why it's important in pharmaceutical manufacturing, what it looks like when used correctly or incorrectly, and common causes when under-developed.
Practical Development
We provide develop-in-place ideas that build skills through real work—not classroom training that doesn't transfer to the manufacturing floor.
How We Develop Leadership Skills
Our leadership development materials provide comprehensive insights into each skill—from why it matters to how to build it through real work.
Why It Matters
Understand the importance of each leadership skill in the context of pharmaceutical manufacturing—connecting skill development to operational performance and strategic execution.
What Good Looks Like
See examples of the skill used correctly in GMP environments—and what it looks like when the skill is under-developed or misapplied.
Common Causes of Gaps
Identify why leaders struggle with specific skills—whether it's lack of training, unclear expectations, or organizational barriers that prevent skill application.
Develop in Place
Build skills through real work assignments, coaching, and structured practice—not generic classroom training that doesn't transfer to operations.
Develop in Place:
We provide practical develop-in-place ideas to build each skill through real work assignments, coaching, and structured practice—not generic classroom training that doesn't transfer to the manufacturing floor or quality operations.
What Makes Our Approach Different
We're not generic leadership consultants. Our development framework is purpose-built for pharmaceutical manufacturing leaders operating in highly regulated GMP environments.
Pharmaceutical Manufacturing Context
Our leadership development framework is designed specifically for GMP environments—addressing the unique challenges of regulated operations, batch manufacturing, and quality oversight.
Level-Specific Development
We identify which skills matter most at each leadership level—ensuring front-line leaders, functional managers, and business unit leaders focus on the right capabilities.
Develop in Place
We build leadership skills through real work, not classroom training. Leaders develop capabilities by applying them to actual operational challenges with coaching support.
Integrated with Strategy Deployment
Leadership development isn't separate from operational improvement—it's integrated with our strategy deployment, diagnostics, and governance systems to ensure skills are applied immediately.
Our Promise:
We develop leaders who can deploy strategy in the context of highly regulated pharmaceutical operations—not just manage day-to-day activities. Our framework builds the critical skills needed to drive operational excellence, execute strategy with discipline, and sustain improvement after we leave.
Related Tools & Resources
Leadership development works alongside other key resources in the GMPKit toolkit.
Ready to Develop Your Leaders?
When your manufacturing network needs leaders who can deploy strategy in highly regulated operations, let's discuss how GMPKit's leadership development framework can build the critical skills your organization needs.
